Best Viewed on Desktop
This website is designed for desktop and may not perform well on mobile.
For a better experience, try viewing in landscape mode.
Lipid-mediated hydrophobic gating in the BK channel
Lucia Coronel, Giovanni Di Muccio, Brad S. Rothberg, Alberto Giacomello & Vincenzo Carnevale
BK channels are large-conductance channels that regulate K⁺ efflux in response to cytosolic Ca²⁺
and membrane depolarization, playing key roles in processes like hearing, neurosecretion,
and smooth muscle contraction. Each channel is a tetramer, with subunits containing
a voltage-sensing domain (VSD:S1–S4 helices),
pore-gate domain (PGD: S5–S6 helices), and
cytosolic tail domain (CTD).
The PGD forms the ion-conducting pore and selectivity filter, while the VSD
detects depolarization, and the CTD binds Ca²⁺. The channel opening can be triggered independently
by either Ca²⁺ binding or voltage sensing
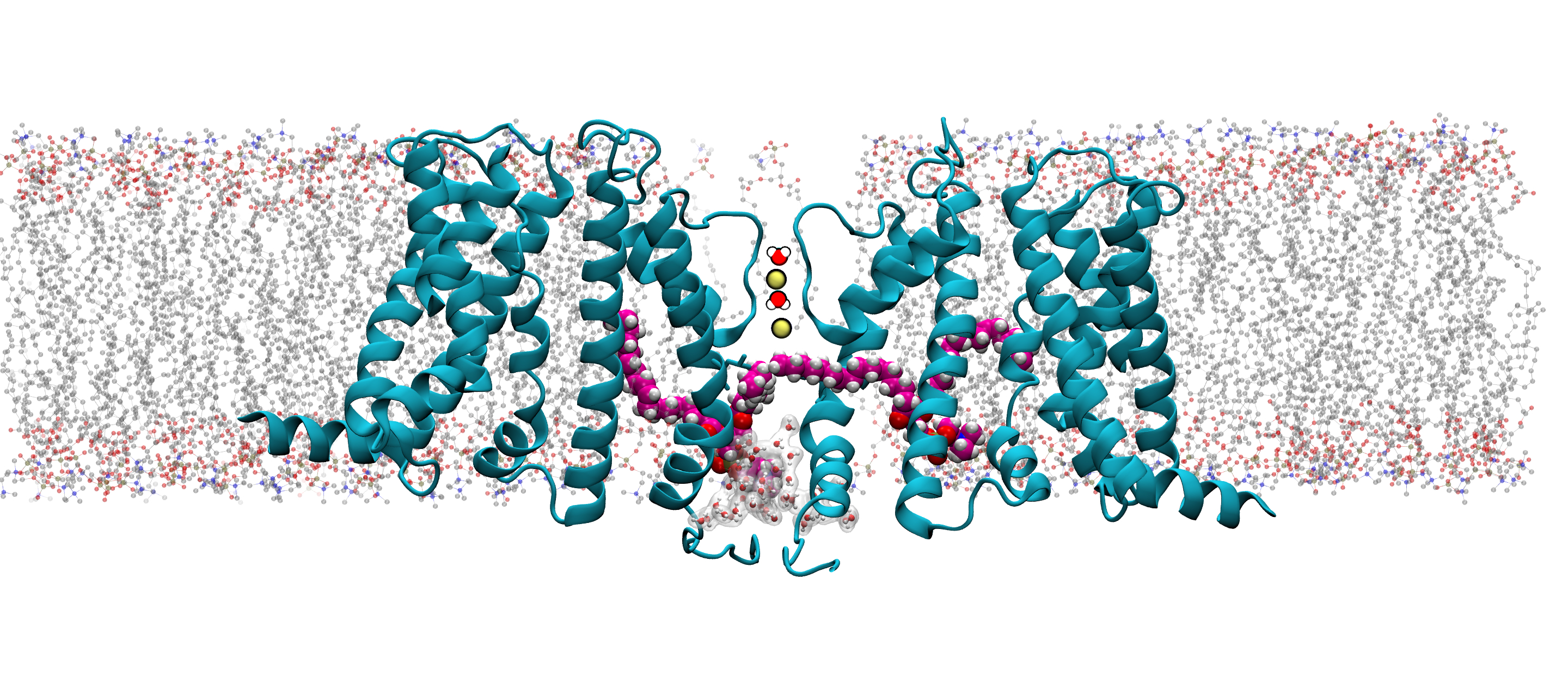
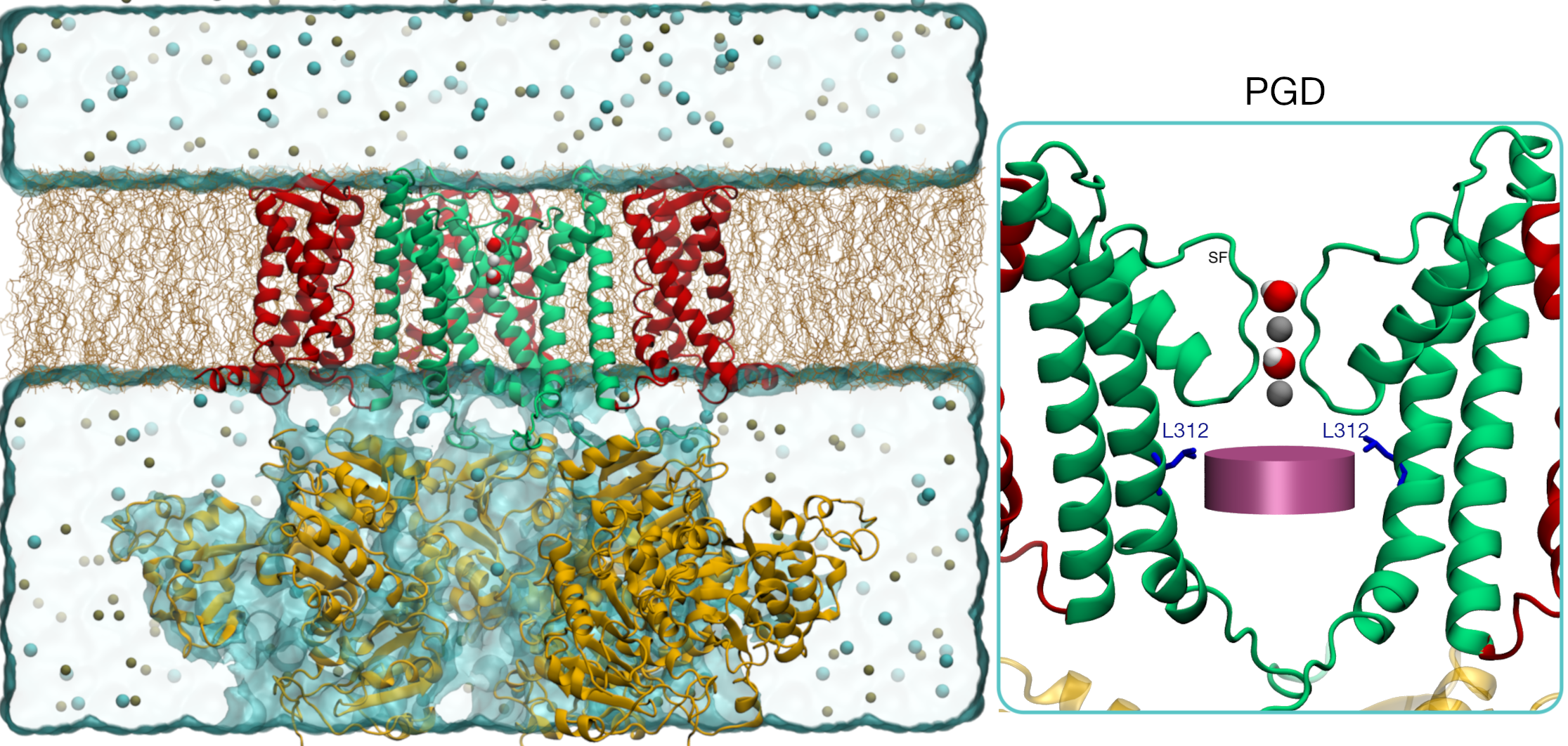
In the inset, the PGD is shown (only two subunits shown for clarity)
along with the K+ ions (gray spheres) and waters occupying the Selectivity-Filter (SF).
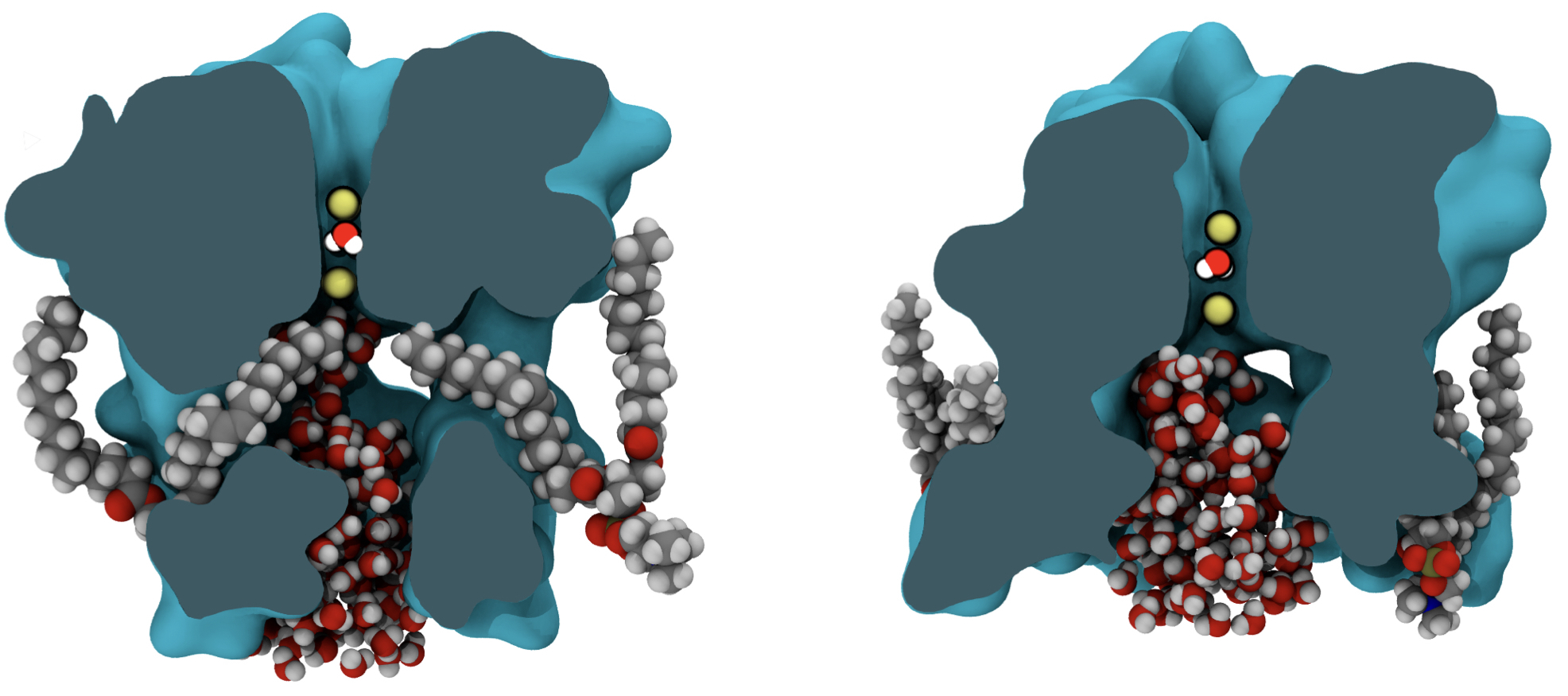
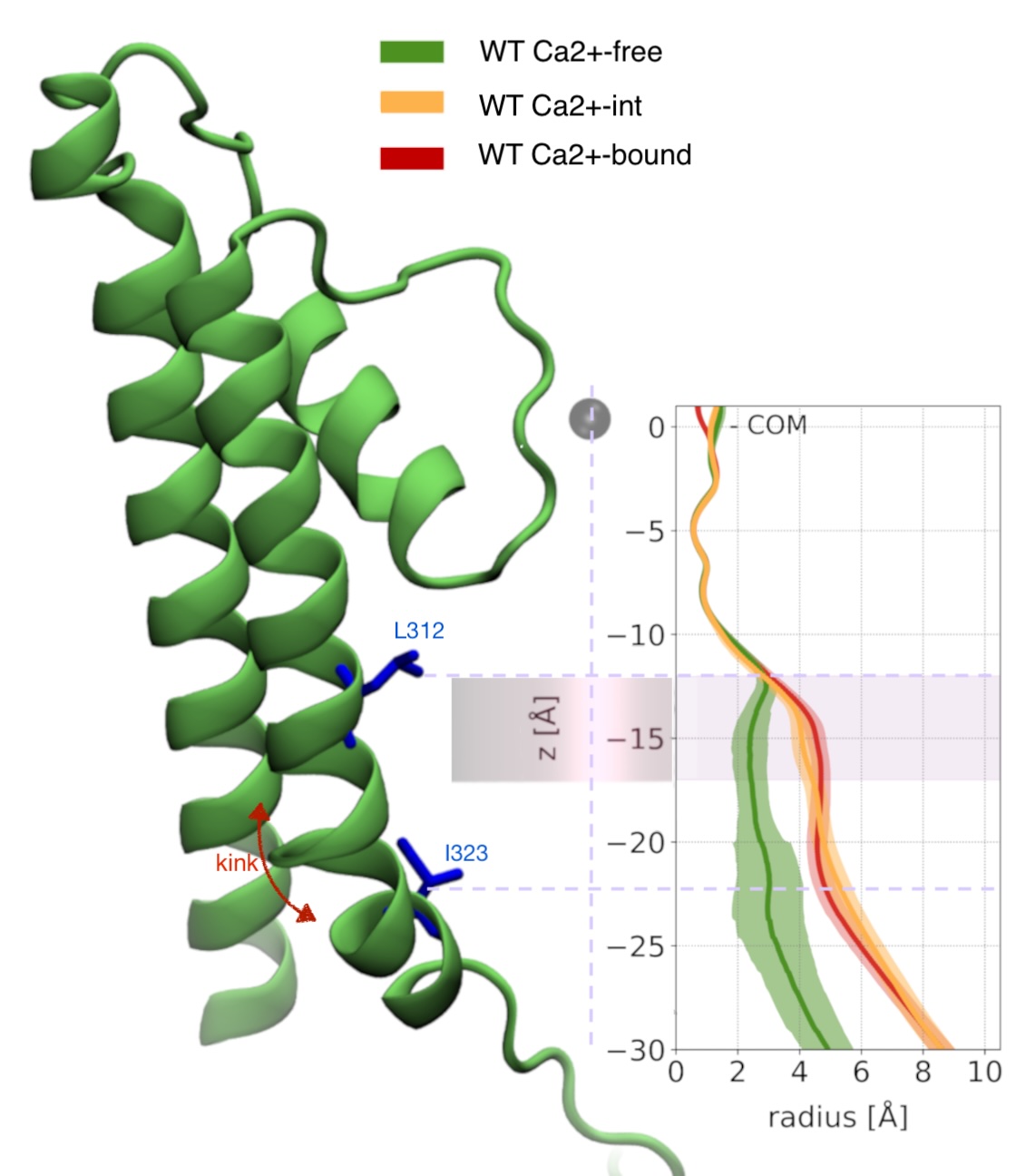
The deep pore volume (DPV) is defined as follows: a cylindrical volume
with a radius of 0.7 nm and
axis aligned to the SF axis of symmetry. The cylinder’s height is 0.5 nm with its topmost circular face
located 1.2 nm below the SF center of mass and the other in proximity of the kink of S6.
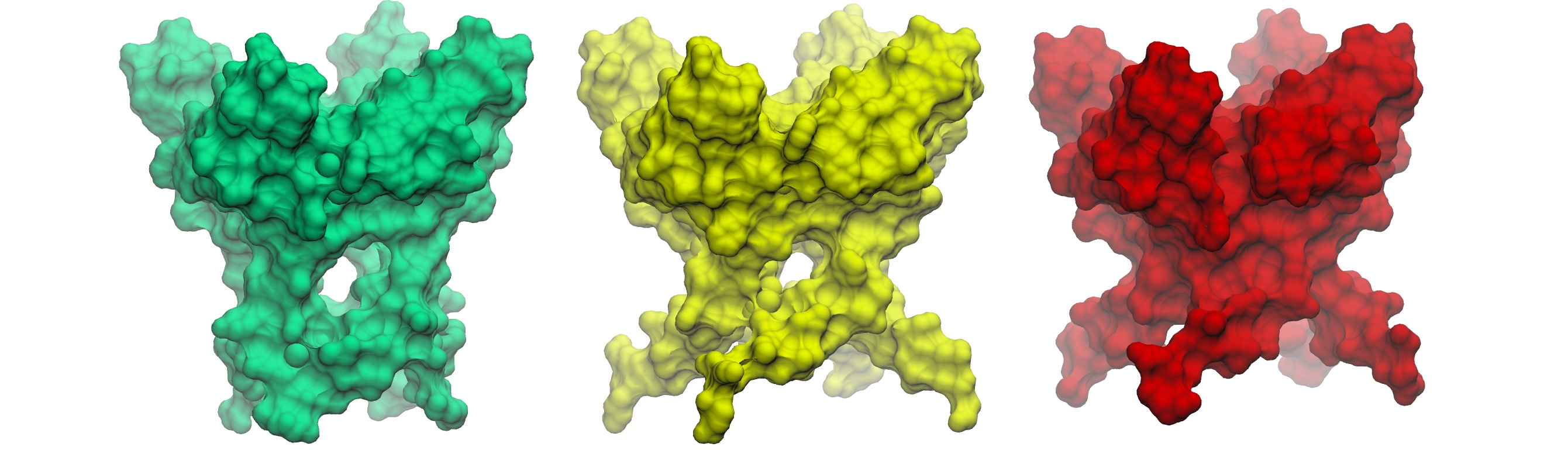
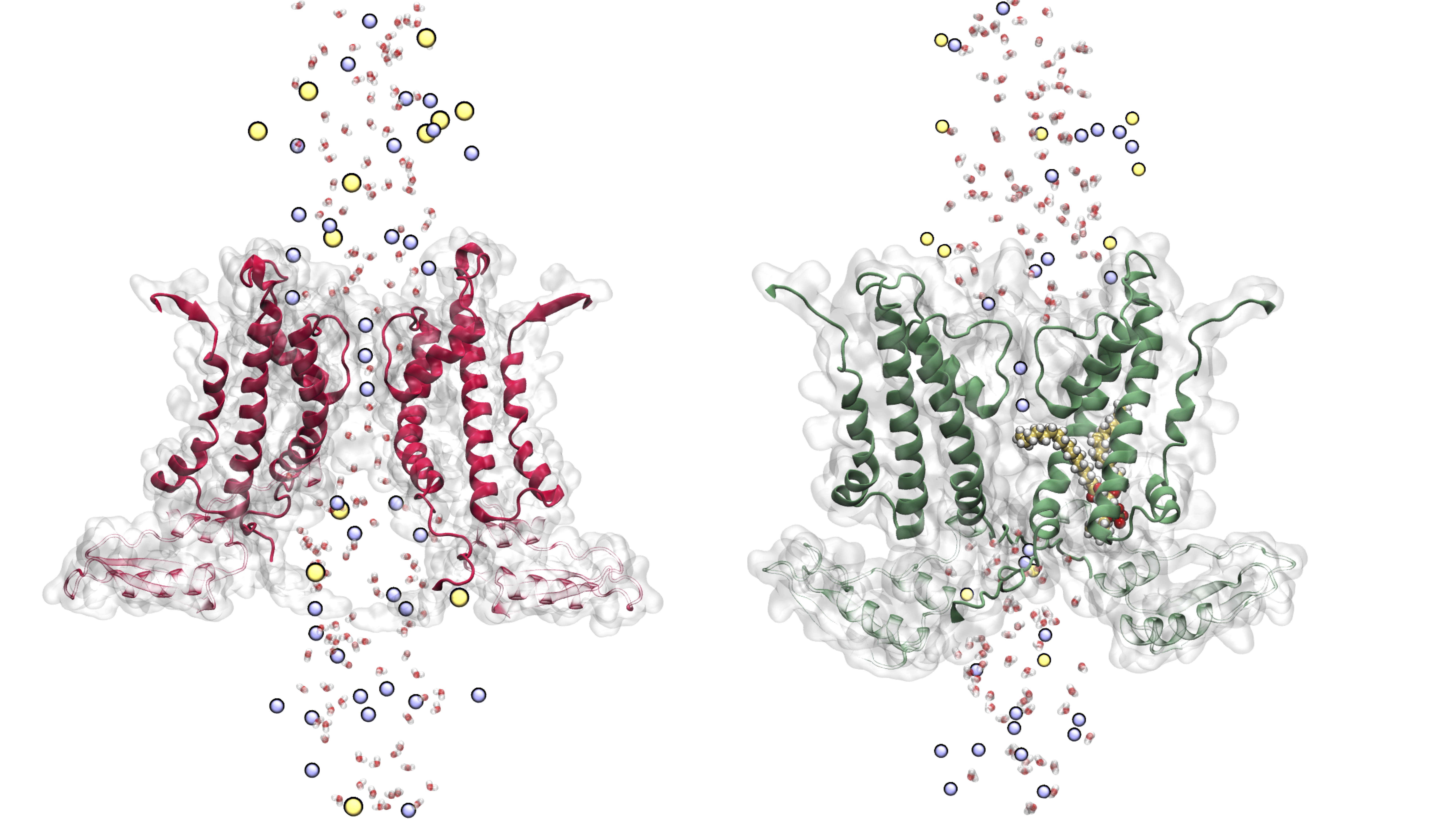
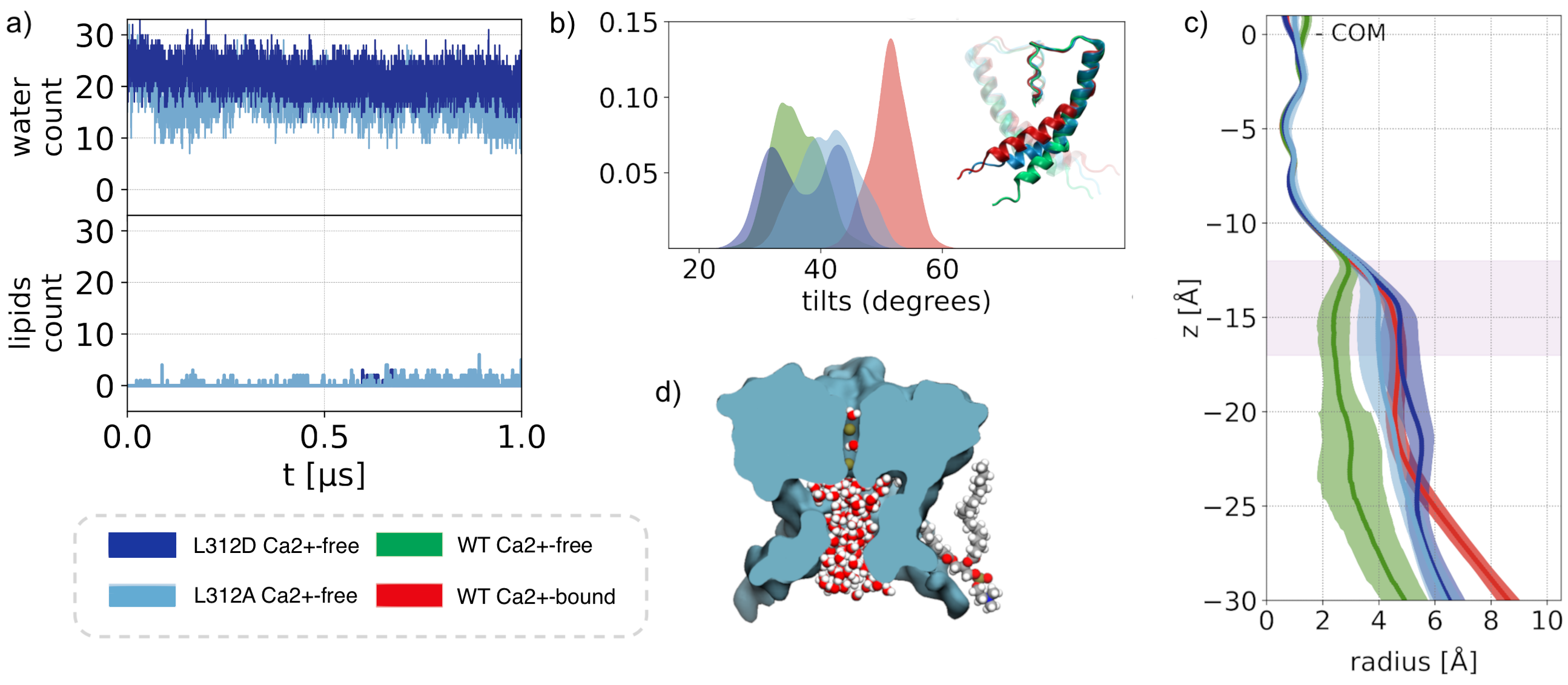
Comparison of three wild-type PGD structures: Ca²⁺-free, Ca²⁺-int, and Ca²⁺-bound. Notice how fenestrations are present in the Ca²⁺-free structure, arising from the kinked conformation of S6.
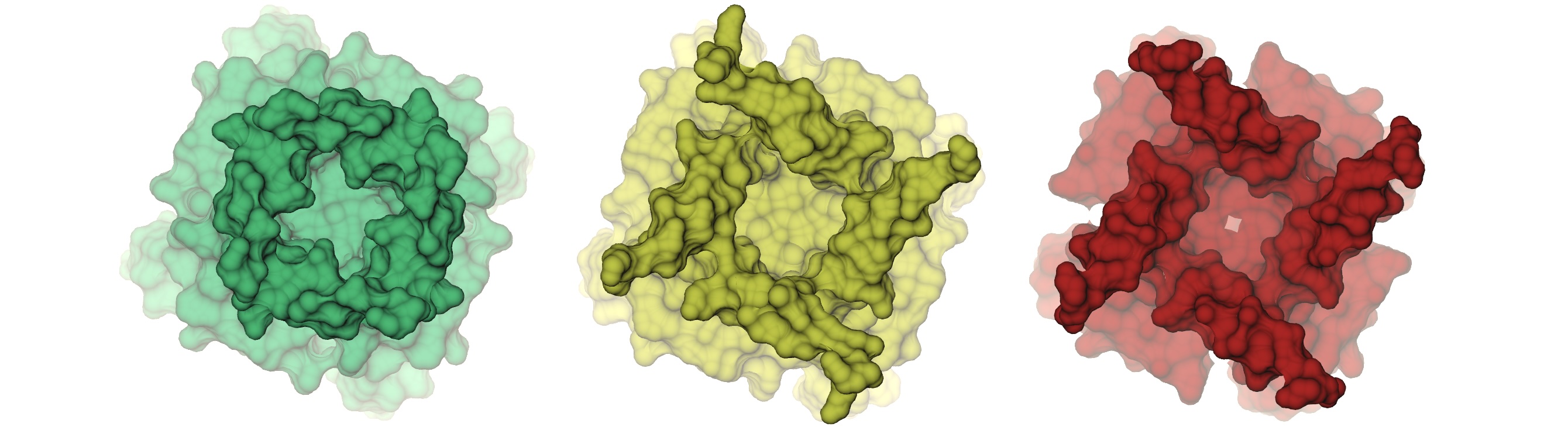
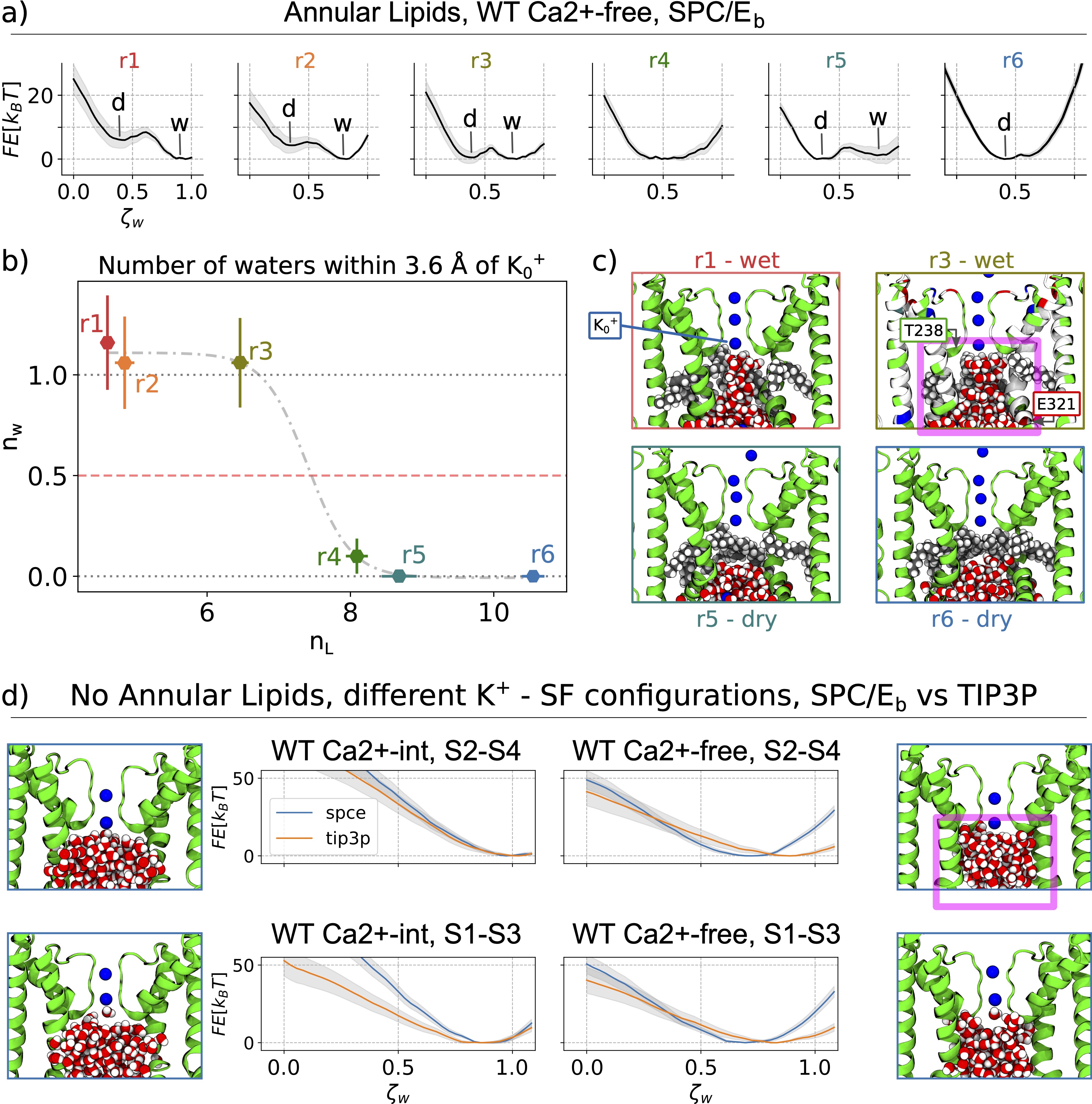
Width of the pore lumen
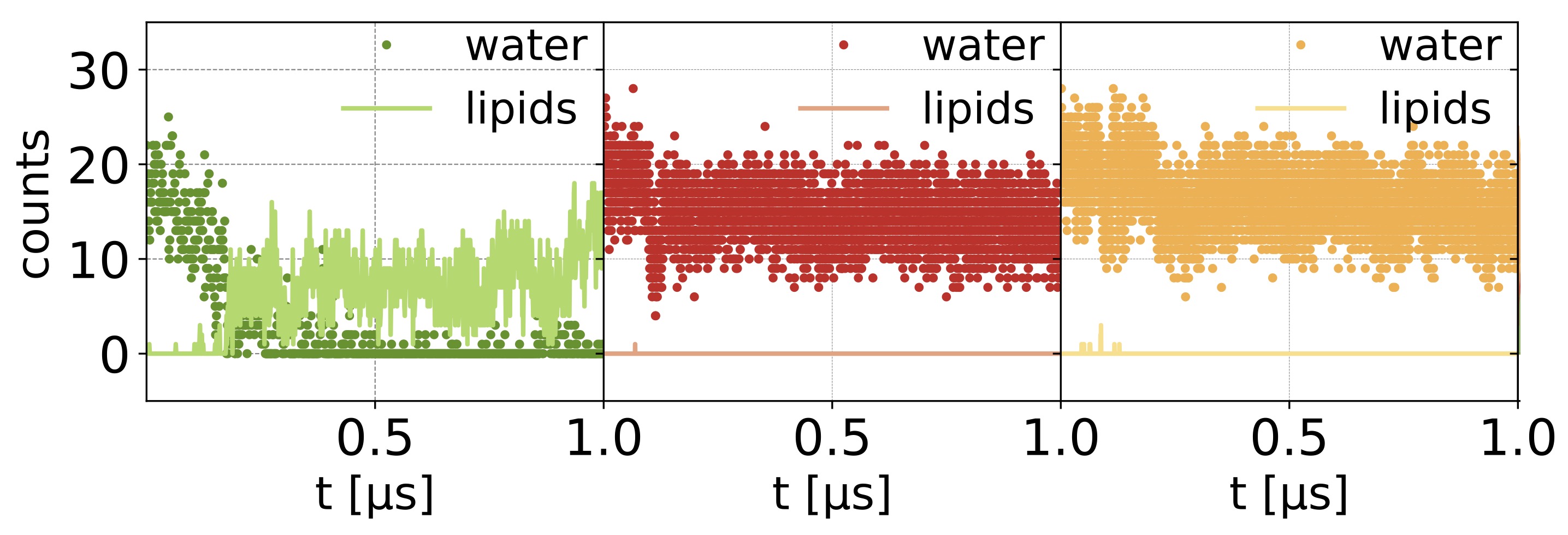
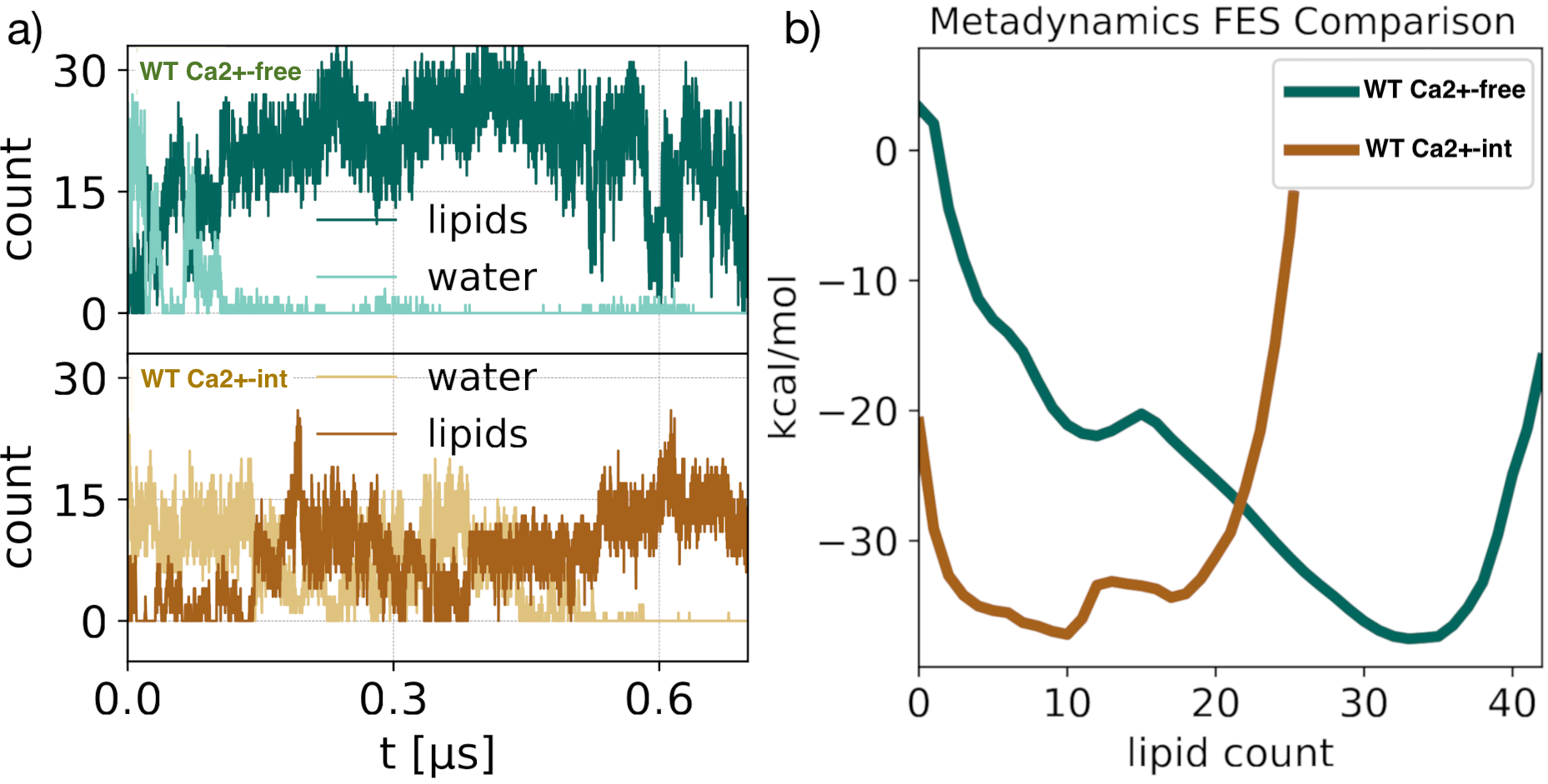
We characterized the channel by computing the pore radius along the axis of symmetry. The conductive (Ca²⁺-bound) and non conductive (Ca²⁺-free) radii begin to branch out at 17 A below the center of mass of the selectivity filter, and keep diverging up to the S6 knk, at z=-17 A, where where the difference between the two pore radii achieves a maximum. We refer to this region as the deep pore volume (DPV). Here, the Ca²⁺-bound radius is always larger than the Ca -free. On the other by Ca²⁺-int structure has a pore lumen radius that resembles the WT Ca²⁺-bound structure Although the pore radii of WT Ca²⁺-bound, WT Ca2+ −int, and WT Ca²⁺-free BK channels are all seemingly large enough to accommodate water and ions, inspection of our molecular simulation trajectories reveals qualitatively different behaviors.
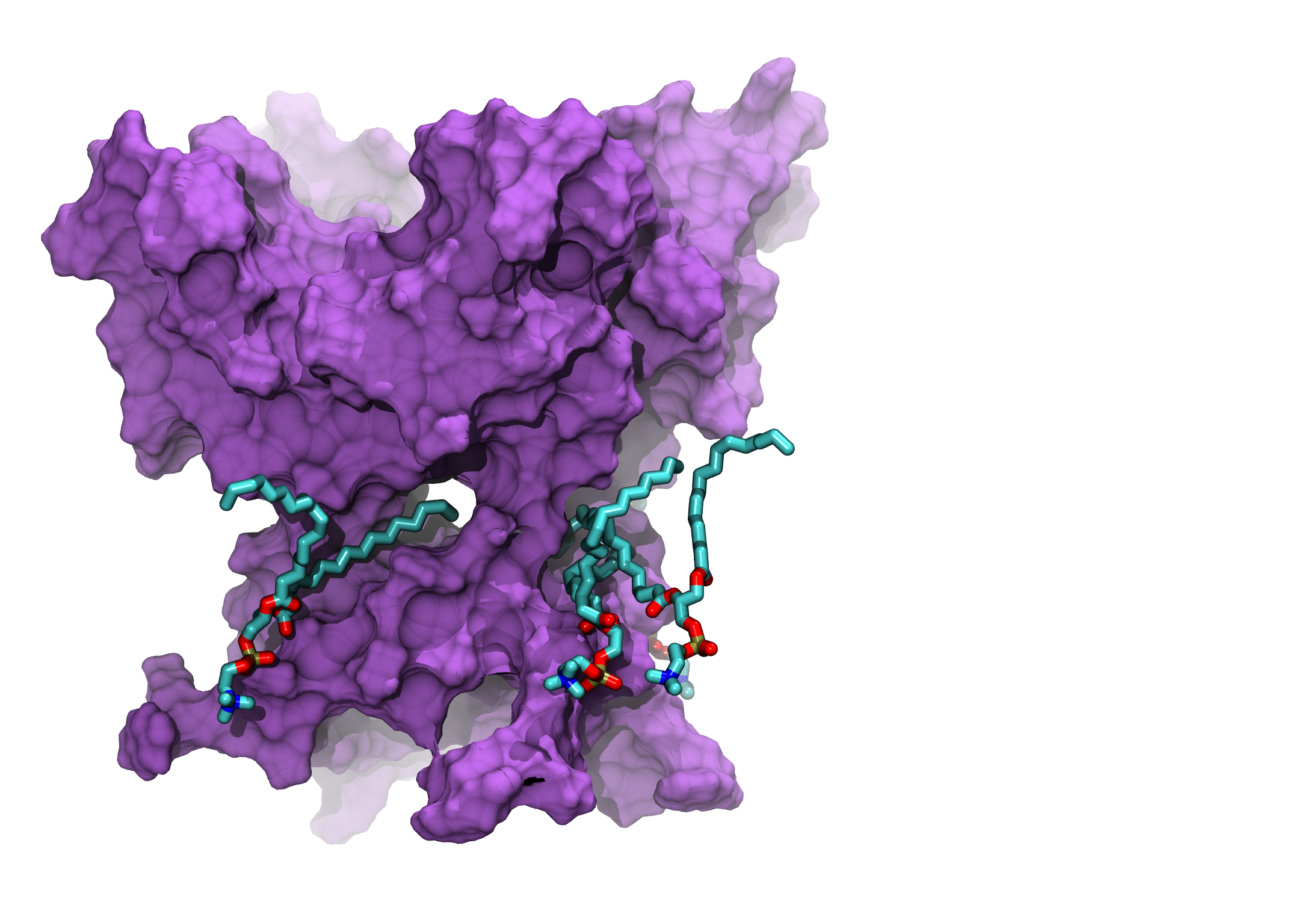
Pore hydration and lipid penetration
- Anticorralation
- Helices Angles distributions
- Steric hindrance by lipid carbon atoms occupying the DPV

Allosteric Coupling
Differences between the WT Ca²⁺-free and WT Ca²⁺-bound state upon considering annular lipid interaction with S6 and the CTD. Upon Ca2+ binding there is a ∼9 Å movement of Lys392 outwards, affecting the chances of lipid tail penetrations. Finally, our simulations show that only the unsaturated tails were able to penetrate the fenestrations; in which the kink favours the penetration.

Gallery
MD simulations (up to 1000ns) of the WT Ca²⁺-free BK Channel, lipids penetrations. Shown PGD only.